Narcolepsy Treatments 2021 Update: Drug Development & Clinical Trials
Drug development in the narcolepsy space has gained incredible momentum the past few years. I shared progress updates in 2018 and 2019, and am excited to publish a new update today!
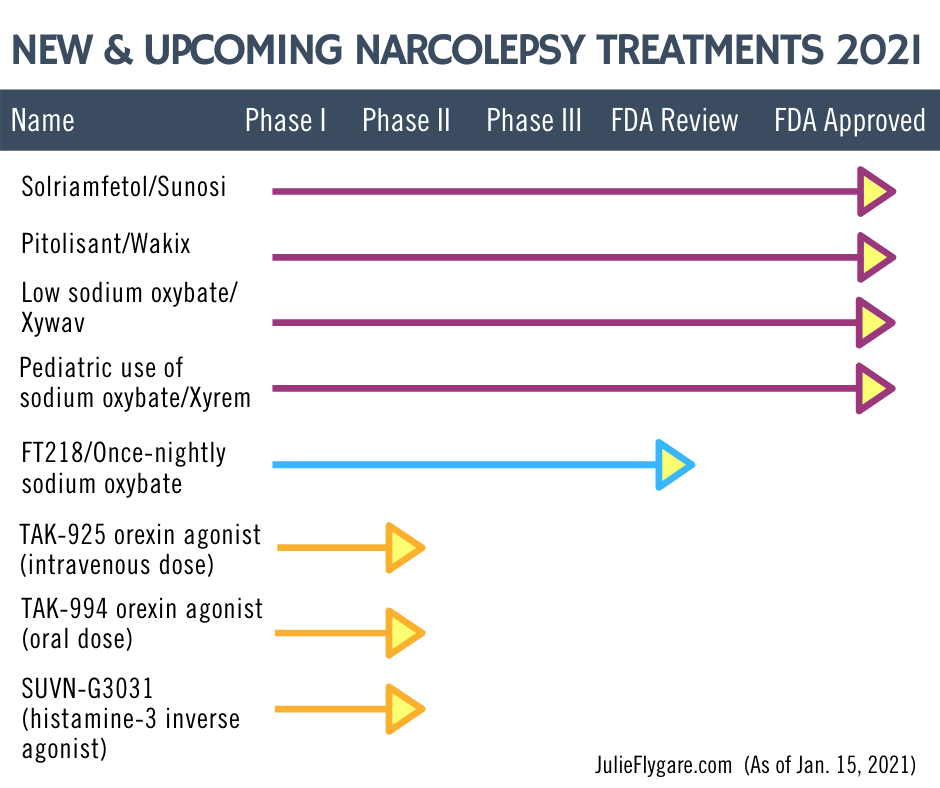
There are new and upcoming narcolepsy treatment options in these categories:
- Wake-promoting or stimulant medications
- Histamine-directed medications
- Nighttime medications
- Hypocretin/orexin agonists
Wake-promoting or stimulant medications
Solriamfetol/Sunosi
Background: Solriamfetol (trade name Sunosi) is a wake-promoting agent, a dual-action dopamine and nonrepinephrine reuptake inhibitor to treat excessive daytime sleepiness in people with narcolepsy and obstructive sleep apnea.
FDA Approval: In March 2019, Jazz Pharmaceuticals announced here that the FDA approved solriamfetol/Sunosi for the treatment of excessive sleepiness in adults with narcolepsy or obstructive sleep apnea. As of July 2019, Sunosi became commercially available in the United States. Learn more.
Histamine-directed medications
Pitolisant/Wakix
Background: Pitolisant is a histamine H3 receptor inverse agonist that activates histamine neurons, which is an exciting advancement because pitolisant works via a different mechanism than other treatment options for narcolepsy. This article offers some ideas about how the treatment may work.
FDA Approval: In 2016, pitolisant (trade name: Wakix) was approved and on the market in Europe. Subsequently, in the U.S., Harmony Biosciences LLC announced FDA approval for pitolisant/Wakix for the indication of excessive daytime sleepiness in narcolepsy in 2019 and the treatment went onto the U.S. market in late 2019. In Oct. 2020, pitolisant/Wakix was also FDA-approved for the indication of cataplexy as well. Learn more.
Further Clinical Research for Prader-Willi Syndrome: As of Dec. 2020, Harmony Biosciences announced that the first patient enrolled in a Phase II trial evaluating the safety and efficacy of pitolisant for the treatment of excessive daytime sleepiness (EDS) and other key symptoms in people with Prader-Willi Syndrome (PWS). Learn more.
SUVN-G3031
Clinical Trial Opportunity: SUVN-G3031 is a novel histamine H3 receptor inverse agonist. Phase II clinical trials are currently underway for people with type 1 narcolepsy with cataplexy and type 2 narcolepsy without cataplexy. I am not familiar with the trial sponsor, Suven Life Sciences, but look forward to learning more about their efforts. Learn more.
Nighttime Medications
Low-sodium oxybate
Background: Xywav is a low-sodium alternative to Xyrem, with 92 percent less sodium per nightly dose. It is an oral solution that is administered at night in two doses.
FDA Approval: In July 2020, Jazz Pharmaceuticals announced that the FDA approved the Xywav oral solution for the treatment of cataplexy or excessive daytime sleepiness in people with narcolepsy who are 7 years of age and older. Xywav is now FDA-approved and available on the market. Learn more.
Pediatric Indication: It is exciting that Xywav was FDA-approved for children ages 7 years and up. This comes soon after Jazz Pharmaceuticals also announced here in 2018 that the FDA approved Xyrem (sodium oxybate) to treat cataplexy and excessive daytime sleepiness (EDS) in children and adolescents with narcolepsy 7 years of age and older.
FT-218
Background: FT218 is a once-nightly formulation of sodium oxybate using Avadel Pharmaceuticals’ proprietary Micropump® technology to provide an extended-release of the drug. This is being studied for the treatment of excessive daytime sleepiness (EDS) and cataplexy in people living with narcolepsy.
Under FDA Review: Since my last update, the FT218 Phase III clinical trial was completed and Avadel reported positive topline data in April 2020. Most recently, in December 2020, Avadel announced the submission of its New Drug Application (NDA) to the FDA.
Clinical Research Opportunity: An open label study is currently evaluating the long-term safety and tolerability of FT218 and the ability to switch from twice-nightly sodium oxybate to once-nightly FT218. Learn more.
Hypocretin/Orexin Agonists
Since 1999, we’ve known that type 1 narcolepsy with cataplexy is caused by a selective loss of neurons producing the neuropeptide orexin (or hypocretin), which plays a central role in maintaining wakefulness. However, finding treatments able to cross the blood-brain barrier and mimic the function of orexin has been scientifically challenging. Over the past 20 years, a few approaches have been explored, but promising research started coming out of Japan a few years ago from Takeda Pharmaceuticals.
TAK-994
Clinical Trial Opportunity: TAK-994 is an oral dose formulation OX2R agonist. Phase II clinical trials are currently underway for people with type 1 narcolepsy with cataplexy and type 2 narcolepsy without cataplexy. Learn more.
TAK-925
Studying Idiopathic Hypersomnia: Takeda is also studying how orexin agonists might work to increase wakefulness in people with idiopathic hypersomnia. The most recent phase II clinical trial examining TAK-925 for people with idiopathic hypersomnia completed enrollment in 2020. I’ll keep you posted as outcomes are results are shared!
Watch this space!
More drug developers are entering the hypocretin/orexin agonists space, including a company called Orexia. The R&D investment from multiple companies is very exciting and gives the narcolepsy community so much hope!
Last but not least….
Medications are essential, yet non-pharmacological approaches are also important to manage symptoms and address social disconnect and stigma. Coping strategies vary by person but may include:
- Scheduled daytime naps
- Social support such as meet-up groups or social media
- Improvement in general health and wellness through sleep hygiene, diet, and fitness
Looking forward to when some of these drugs will actually be accessible to Canadians.
My 26 year old daughter has narcolepsy with excessive day time sleepiness. She lives on her own with a room mate and works as an RN in a hospital. I need to better understand as well as better support her and her older sister .
Thanks
A concerned Mom
Thank you for supporting your daughter’s experience with narcolepsy. I know I’m very biased but I have heard from a lot of family members of people with narcolepsy that my memoir was a helpful starting point, and that the loved one could use the book to then ask questions of the person with narcolepsy in their life to see how their experience might be similar or different. Again, I’m biased and wouldn’t mention it but even my own mom found this useful! 🙂 Check out some reviews on Amazon to see if this might be something useful for you.
Thank you for this post. It is a good reminder to pursue potentially more effective medications. I haven’t done any research on the ones related to histamine, but I’m curious if they would be helpful or unhelpful for those with the heterozygous comt mutation.
I second Julie’s book! It was a great resource that I wish I had found earlier. I felt as though I was reading my own story! It really gave me comfort to know that I’m not alone in this crazy journey! Wishing you the best as you help your daughter navigate this disease. She is blessed to have you in her corner!
With kindness,
Rachael