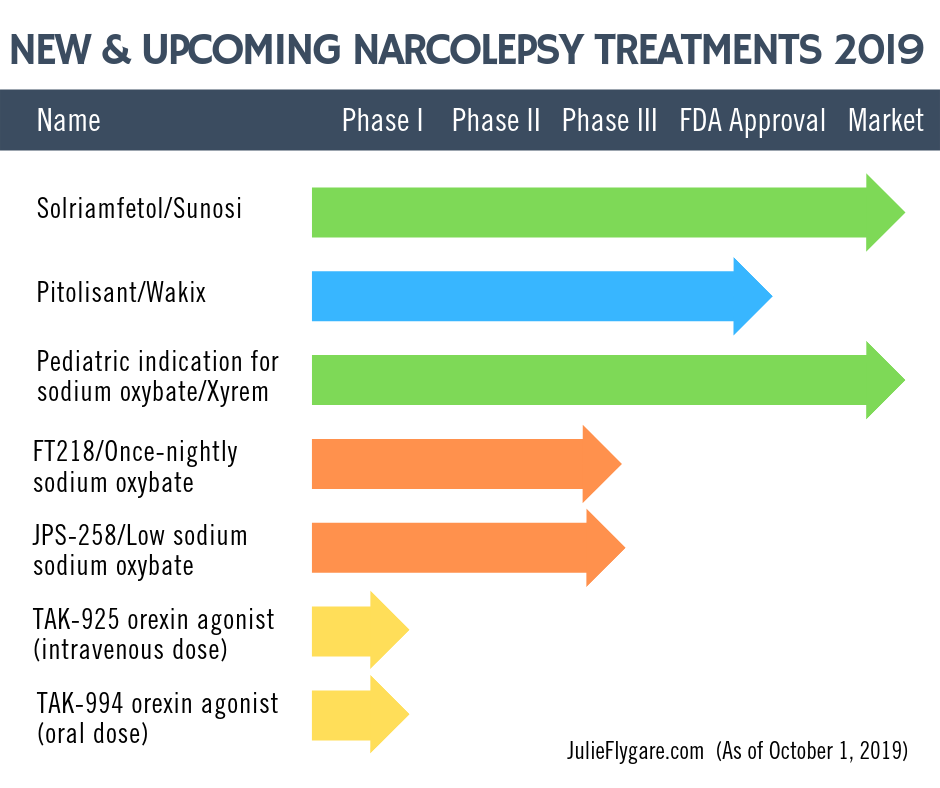
New and Upcoming Treatments for Narcolepsy 2019! Part II Update from World Sleep

Did you know the FDA has approved 27 novel drugs so far in 2019, and two of those are for narcolepsy?! How amazing is that?! The diversification of treatment approaches and new therapies under development and gaining FDA-approval for narcolepsy in the U.S. is SO EXCITING!
In this post, I will provide an update on the narcolepsy drug development space and report back highlights from World Sleep 2019. This is Part II, Part I on hypocretin/orexin agonists is here.
Recent FDA Approvals:
Solriamfetol/Sunosi
- Background: Solriamfetol (trade name Sunosi) is a wake-promoting agent, a dual-action dopamine and nonrepinephrine reuptake inhibitor to treat excessive daytime sleepiness in people with narcolepsy and obstructive sleep apnea. Clinical and preclinical data suggested that the wake-promoting effects of solriamfetol differ from medications such as modafinil and amphetamine.
- Research Findings: Approval of Sunosi was based on data from clinical trials including:
- One study evaluating excessive sleepiness in adult patients with narcolepsy (TONES 2)
- Two studies evaluating excessive sleepiness in adult patients with OSA (TONES 3 and TONES 4),
- An open-label long term safety and maintenance of efficacy for the treatment of excessive sleepiness in patients with narcolepsy or OSA (TONES 5).
- FDA APPROVAL & Available Now: On March 20, 2019, Jazz Pharmaceuticals announced here that the FDA approved solriamfetol/Sunosi for the treatment of excessive sleepiness in adults with narcolepsy or obstructive sleep apnea. As of July 2019, Sunosi is now commercially available for healthcare professionals and patients in the U.S.
Pitolisant/Wakix
- Background: Pitolisant is a histamine H3 receptor inverse agonist that activates histamine neurons, which is an exciting advancement because pitolisant works via a different mechanism of action than any other treatment options currently available for narcolepsy. This article offers some neat ideas about how the treatment may work.
- Pitolisant has been on the market in Europe since 2016 when approved by the European Medicines Agency. In Europe, pitolisant is known by its trade name “Wakix”. In October 2017, Harmony Biosciences, LLC acquired the rights to develop, register and market the drug in the United States.
- Research Findings:
- Phase 3 study results focused on excessive daytime sleepiness: https://www.ncbi.nlm.nih.gov/pubmed/24107292
- Subsequent Phase 3 study results focused on cataplexy: https://www.ncbi.nlm.nih.gov/pubmed/28129985
- At World Sleep 2019: In an oral presentation, Dr. Jeffrey Dayno of Harmony Biosciences shared the results from a study evaluating the abuse potential of pitolisant compared with the stimulant phentermine HCl (C-IV) and placebo in non-dependent, recreational stimulant users. Pitolisant had much lower scores on drug liking compared to phentermine consistent with a lower abuse potential than the more traditional stimulant. Harmony also presented posters further highlighting the efficacy and safety of pitolisant overtime. Read more.
- FDA APPROVAL and Available Soon: On August 15, 2019, Harmony Biosciences, LLC announced here that the U.S. Food and Drug Administration (FDA) approved pitolisant (also U.S. commercial name “Wakix”) for the treatment of excessive daytime sleepiness (EDS) in adults with narcolepsy. The treatment will become commercially available to healthcare professionals and patients in the U.S. starting in the fourth quarter of 2019!
- Important: Wakix may decrease the effectiveness of hormonal birth control. Women should know this information and discuss with their doctors.
Pediatric indication for sodium oxybate/Xyrem
- Background: While some children previously took sodium oxybate to treat cataplexy and excessive daytime sleepiness of narcolepsy, it was considered “off-label” use because it had not been formally evaluated and FDA-approved specifically for a pediatric population.
- Research Findings: E Mignot, G Plazzi, Y Dauvilliers, C Rosen, C Ruoff, J Black, R Parvataneni, D Guinta, Y Wang, M Lecendreux; 0813 Sodium Oxybate Treatment of Narcolepsy in Pediatric Patients: Long-term Efficacy and Safety, Sleep, Volume 41, Issue suppl_1, 27 April 2018, Pages A302, https://doi.org/10.1093/sleep/zsy061.812
- FDA APPROVAL: On October 29, 2018, Jazz Pharmaceuticals announced here that the FDA approved Xyrem (sodium oxybate) to treat cataplexy and excessive daytime sleepiness (EDS) in children and adolescents with narcolepsy ages seven and older.
Novel Narcolepsy Treatments in Development:
FT218/Once-nightly formulation of sodium oxybate
- Background: FT218 is a once-nightly formulation of sodium oxybate using Avadel Pharmaceuticals’ proprietary Micropump® technology to provide an extended-release of the drug. It is currently undergoing testing in a Phase 3 clinical trial for the treatment of excessive daytime sleepiness (EDS) and cataplexy in people living with narcolepsy.
- Research Findings:
- First-in-Human Study
- Second Clinical Trial Results
- At World Sleep 2019: Dr. Michael Thorpy shared the latest research findings: The Pharmacokinetics of once-nightly controlled-release sodium oxybate (FT218): Overview of results from four Phase 1 Studies.
- Current status: On Sept. 23, 2019, Avadel announced here that the current phase 3 REST-ON clinical trial should be fully enrolled by the end of 2019.
- Get Involved: As of now, the Phase 3 REST-ON clinical trial is still enrolling people with narcolepsy with cataplexy in the U.S. and internationally. For more information, visit http://clinicaltrial.avadel.com/ and ClinicalTrials.gov Identifier: NCT02720744
JPS-258/Low sodium version of sodium oxybate
- Background: JZP-258 is a low-sodium version of sodium oxybate that has 92 percent less sodium. Phase 3 clinical trials were recently conducted.
- Update from World Sleep 2019: On Sept. 25, Jazz Pharmaceuticals announced here that the phase 3 clinical trials for JZP-258 achieved primary and key secondary endpoints demonstrating reductions of cataplexy and improved wakefulness via Epworth Sleepiness Scale scores compared to placebo.
- Current Status: Jazz aims to submit the New Drug Application as early as the end of this year (2019).
Notes & Thanks!
- This is NOT a full list of all emerging treatments under development for narcolepsy. For a more thorough review of emerging treatments from March 2017: New developments in the management of narcolepsy.
- I am not a scientist or doctor. You should always speak with your narcolepsy specialist about treatment options and whether a clinical trial would be a good option for you. However, I hope this post helps provide access to information, because when navigating the healthcare system with a serious condition like narcolepsy, information is power.
- Thank you to all those who are working to make this progress possible: scientists, drug developers, clinical research teams, and individuals with narcolepsy and families around the world. Your contributions make real difference.
Watch this space! While this post is accurate as of October, 1, 2019, things move rapidly. Be sure to subscribe to my blog (top right corner) and follow me on socials to get the latest updates.
Part I of this post on hypocretin/orexin agonists!