Unite Narcolepsy Interim Survey Results Paint Honest Picture of Challenges Facing Narcolepsy Community
In just six week, the Unite Narcolepsy’s patient survey has collected over 1,350 responses, including over 1,000 responses directly from patients diagnosed with narcolepsy. The survey results paint a powerful picture of the challenges facing the narcolepsy community – including extensive delays in diagnosis and many unmet medical needs. The Interim Survey Analysis will be shared at FDA’s Narcolepsy Patient Focused Meeting next Tuesday, Sept. 24, 2013 in Silver Spring, MD. [View/Download Results Here.]
Key Results Include:
-
 Cataplexy, a striking and sudden episode of muscle weakness often triggered by strong emotion, was reported by 65% of respondents. The three symptoms rated as having the most significant impact on patients’ lives were excessive daytime sleepiness (77%), difficulty thinking, remembering, concentrating or paying attention (50%), and general fatigue/never feeling rested (45%).
Cataplexy, a striking and sudden episode of muscle weakness often triggered by strong emotion, was reported by 65% of respondents. The three symptoms rated as having the most significant impact on patients’ lives were excessive daytime sleepiness (77%), difficulty thinking, remembering, concentrating or paying attention (50%), and general fatigue/never feeling rested (45%). -
Nearly 95% of survey respondents reported having been prescribed one or more of the four medications approved by FDA for treatment of narcolepsy or its key symptoms (Adderall, Nuvigil, Provigil, and Xyrem). 70% use other prescription medications (including stimulants, anticataplectics, and hypnotics/sedatives), and 80% use other therapies (such as lifestyle modifications, nutritional supplements, and diet) to help manage their condition. Fewer than 8% reported that they currently pursue no form of treatment for the condition.
-
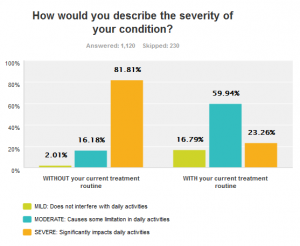 Even with available treatments, the impact of narcolepsy on daily life is profound. 83% report not being able to perform as they wish at work or in school. 76% indicate that they have difficulty interacting with family or friends, and 70% can’t get through the day without falling asleep.
Even with available treatments, the impact of narcolepsy on daily life is profound. 83% report not being able to perform as they wish at work or in school. 76% indicate that they have difficulty interacting with family or friends, and 70% can’t get through the day without falling asleep. -
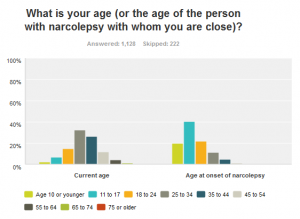
Nearly 51% of survey respondents reported that it took 6 years or longer to get properly diagnosed
**I highly recommend reading the full report here – it left me in tears.**
The survey remains open til Nov. 11, 2013 – and final results will be reported to the FDA through the docket system on November 25, 2013. Start survey here: https://www.surveymonkey.com/s/unitenarcolepsy.
Thank you for participating and making this survey such a powerful tool to help raise awareness about narcolepsy at FDA and in many other settings!
Julie,
Thank you so much for posting this on your blog. My computer will not let me open it and I’ve been dying to know what it said!
Thank you again for all you do for us 🙂
Was interesting reading some of the comments, they matched the kind of thing I thought of….though I almost never leave comments in surveys … even if it makes it a required element.
The interim report doesn’t show it, but I’d be curious to see if shows the distribution of time to diagnosis, and correlates age and year of diagnosis.
Because the doctor that diagnosed me with Cataplexy, didn’t know what Cataplexy was two years earlier….
Thanks so much for posting some of the results. I just can’t read the whole thing. Narcolepsy has completely stolen my attention span for reading.
Thank you Julie!
The most interesting fact to emerge (in my opinion) is that a full 60% of respondents claim that the onset of symptoms was before age 18 (20% before age 10, 40% between age 11-17), yet NOT ONE SINGLE medication to treat narcolepsy is approved for use in the pediatric population! This is completely unacceptable and needs to be addressed so our young children with narcolepsy can lead a full, active and productive childhood (socially, academically, physically) that can fully prepare them for their future!
~ Mom to an 8 yr old daughter with severe narcolepsy and cataplexy, living with the challenges of childhood narcolepsy without an effective treatment.
[…] launched this effort over a year ago. Thank you to the Unite Narcolepsy team for launching the powerful survey and successful webinar series. FDA has been “wowed” by our community’s […]