Orexin/Hypocretin Agonists are Coming! Part I: Reporting Back from World Sleep 2019
Room 116: A Glimpse of the Future
It was a basic, boring conference room, but there was nothing basic or boring about Room 116 at the Vancouver Convention Center on Wednesday, Sept. 25, 2019 at 4:30 p.m. As the final session of the final of five days of the World Sleep Congress including World Narcolepsy Day, I felt wobbly and cataplectic, but I couldn’t go home, not yet.
At exactly 4:30 p.m., I eagerly stepped into Room 116 to join narcolepsy experts, drug developers and other patient advocates for a look into the future.
This final session, simply titled “Narcolepsy” – included researchers sharing important new results related to novel and upcoming narcolepsy therapies. In July 2018, I reported here on the unprecedented amount of drug development underway for narcolepsy. Since then, the FDA has made three important approvals and more drug development continues on, full-steam ahead!
So it’s time for an update. Part I will catch you up to speed on orexin/hypocretin agonists, and Part II will share many more exciting developments.
Dr. Todd Swick shares his excitement for new drugs to treat narcolepsy:
Advancing Orexin-Related Therapies
Since 1999, we’ve known that type 1 narcolepsy with cataplexy is caused by a selective loss of neurons producing the neuropeptide orexin (or hypocretin), which plays a central role in maintaining wakefulness. However, finding compounds able to cross the blood-brain barrier and mimic the function of orexin has been scientifically challenging. Over the past 20 years, a few approaches have been explored, but promising research started coming out of Japan a few years ago with Takeda Pharmaceuticals.
So when Dr. Rebecca Evans took the stage in Room 116 to present Takeda’s first-in-human clinical trial findings for the orexin 2 receptor selective agonist, TAK-925, the excitement was palpable. I felt honored to be in the room as these outcomes were shared publicly for the first time here.

TAK-925
- Background: In 2017, a patent for orexin 2 receptor selective agonists, including the clinical candidate TAK-925 was claimed. In April 2018, the orexin 2 receptor-selective agonist, TAK-925, was found to significantly increase wakefulness and recover cataplexy-like episodes in mice with a type of narcolepsy.
- A second publication reported on the pharmacological and electrophysiological characterization of TAK-925 from in vitro (petri dish-type studies) and in vivo (studies in wild-type mice, common marmosets, and cynomolgus monkeys). TAK-925 was shown to induce wake-promoting effects in the mice and nonhuman primates.
- Research Findings:
- T Fujimoto, K Rikimaru, K Fukuda, H Sugimoto, T. Matsumoto, N Tokunaga, M. Hirozane SUBSTITUTED PIPERIDINE COMPOUND AND USE THEREOF WO2017135306 https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017135306
- M Suzuki, H Yukitake, T Ishikawa, H Kimura; 0001 An Orexin 2 Receptor-selective Agonist TAK-925 Ameliorates Narcolepsy-like Symptoms In Orexin/ataxin-3 Mice, Sleep, Volume 41, Issue suppl_1, 27 April 2018, Pages A1, https://doi.org/10.1093/sleep/zsy061.000
- H Yukitake, T Ishikawa, A Suzuki, Y Shimizu, M Nakashima, T Fujimoto, K Rikimaru, M Ito, M Suzuki, H Kimura; 0002 An Orexin 2 Receptor-selective Agonist, TAK-925, Shows Robust Wake-promoting Effects In Mice And Non-human Primates, Sleep, Volume 41, Issue suppl_1, 27 April 2018, Pages A1, https://doi.org/10.1093/sleep/zsy061.001
- New findings shared at World Sleep 2019: Dr. Rebecca Evans of Takeda Pharmaceuticals shared the highly-anticipated results of the first-in-human phase 1 clinical trial of TAK-925 in people with narcolepsy, healthy adults, and elderly people.
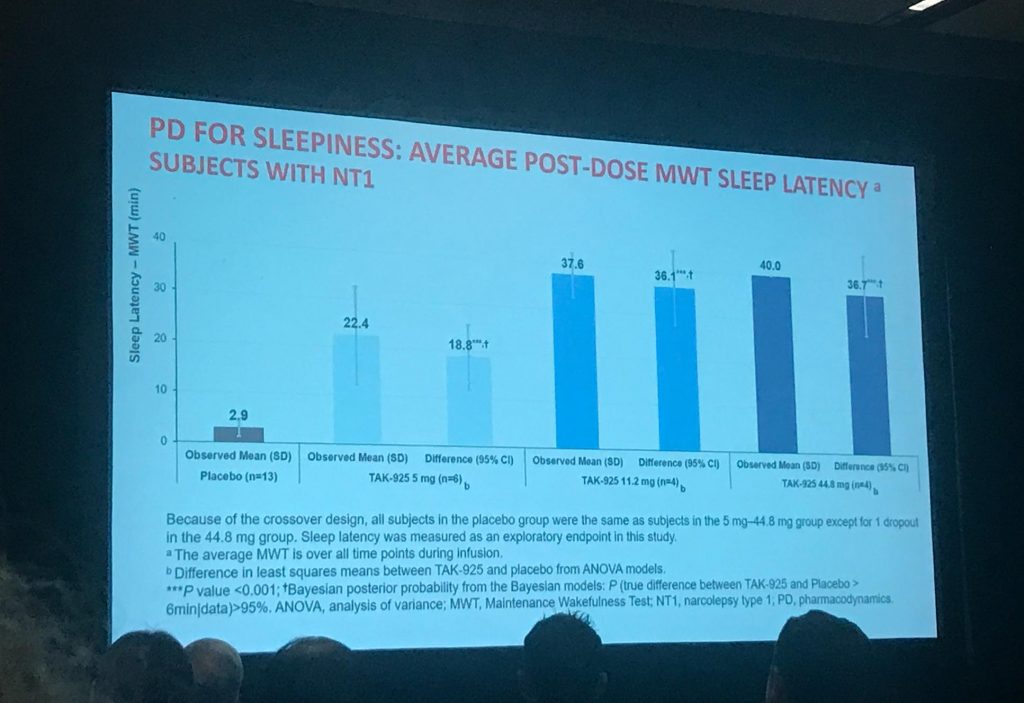
- The study measured TAK-925’s impact on people with type 1 narcolepsy’s wakefulness by a few measures, but the one that stuck out to me was the “Maintenance of Wakefulness Test” (MWT) data. The MWT is a fancy name for “stare at a blank wall for 40 minutes and see how long before you to fall asleep.” In this study, when the people with type 1 narcolepsy received a “placebo” infusion (a.k.a. no real drug), they stayed awake for an average of 2.9 mins out of the total 40 mins. When participants had various doses of TAK-925, they stayed awake much longer, averaging:
- 22.4 mins with TAK-925 at 5mg
- 37.6 mins with TAK-925 at 11.2mg
- 40 mins with TAK-925 at 44.8mg
- The study measured TAK-925’s impact on people with type 1 narcolepsy’s wakefulness by a few measures, but the one that stuck out to me was the “Maintenance of Wakefulness Test” (MWT) data. The MWT is a fancy name for “stare at a blank wall for 40 minutes and see how long before you to fall asleep.” In this study, when the people with type 1 narcolepsy received a “placebo” infusion (a.k.a. no real drug), they stayed awake for an average of 2.9 mins out of the total 40 mins. When participants had various doses of TAK-925, they stayed awake much longer, averaging:
- New findings shared at World Sleep 2019:A Phase 1B trial of TAK-925 in sleep-deprived healthy adults in the US showed wake-promoting effects even though these individuals presumably have normal orexin levels. Importantly, these findings have encouraged Takeda to consider testing orexin agonists for people who are sleepy but don’t have an orexin/hypocretin deficiency, a.k.a. people with type 2 narcolepsy without cataplexy, idiopathic hypersomnia, or residual excessive daytime sleepiness due to obstructive sleep apnea.
- Current Status:
- Not yet enrolling, but now listed on clinicaltrials.gov: a Phase 1b clinical trial in the U.S. and Japan will evaluate TAK-925 in people with idiopathic hypersomnia (IH). To learn more, see https://clinicaltrials.gov/ct2/show/NCT04091438 and http://www.ox2rsparkleprogram.com.
- Not yet enrolling, but now listed on clinicaltrials.gov: a Phase 1b clinical trial in the U.S. will evaluate TAK-925 in adults with obstructive sleep apnea (OSA) and who are experiencing excessive daytime sleepiness (EDS). To learn more, see https://clinicaltrials.gov/ct2/show/NCT04091425 and http://www.ox2rsparkleprogram.com.
TAK-994 – An Oral Formulation!
- Background: While TAK-925 uses IV infusions, Takeda is preparing to investigate an oral dose formulation OX2R agonist, TAK-994.
- Research findings:
- Two posters at the World Sleep Congress described that TAK-994 was studied in two mouse models of narcolepsy and increased wakefulness and suppressed cataplexy-like episodes.
- Current Status: Not yet enrolling, but now listed on clinicaltrials.gov: a Phase 1 clinical trial in North America and Japan to evaluate oral doses of TAK-994 in people with Type 1 Narcolepsy with Cataplexy. To learn more, see https://clinicaltrials.gov/ct2/show/NCT04096560 and http://www.ox2rsparkleprogram.com.
I also found it very powerful and meaningful that Dr. Evans took the time at the end of her presentation to say, “Thank you to the study participants, especially those with type 1 narcolepsy with cataplexy who washed out of their medications to participate in this study.”

In my work with the White House and Stanford MedX on Precision Medicine and Participant Engagement, I’d felt that genuine recognition of the people who participate in research goes a long way toward making research a more respectful process.
(Please note: I am not a scientist or doctor. You should always speak with your narcolepsy specialist about treatment options and whether a clinical trial would be a good option for you. However, I hope this post helps provide access to information, because when navigating a complex healthcare system with a serious condition like narcolepsy, information is power.)
Stay tuned! I will follow up with Part II soon sharing more advancements in narcolepsy treatments.